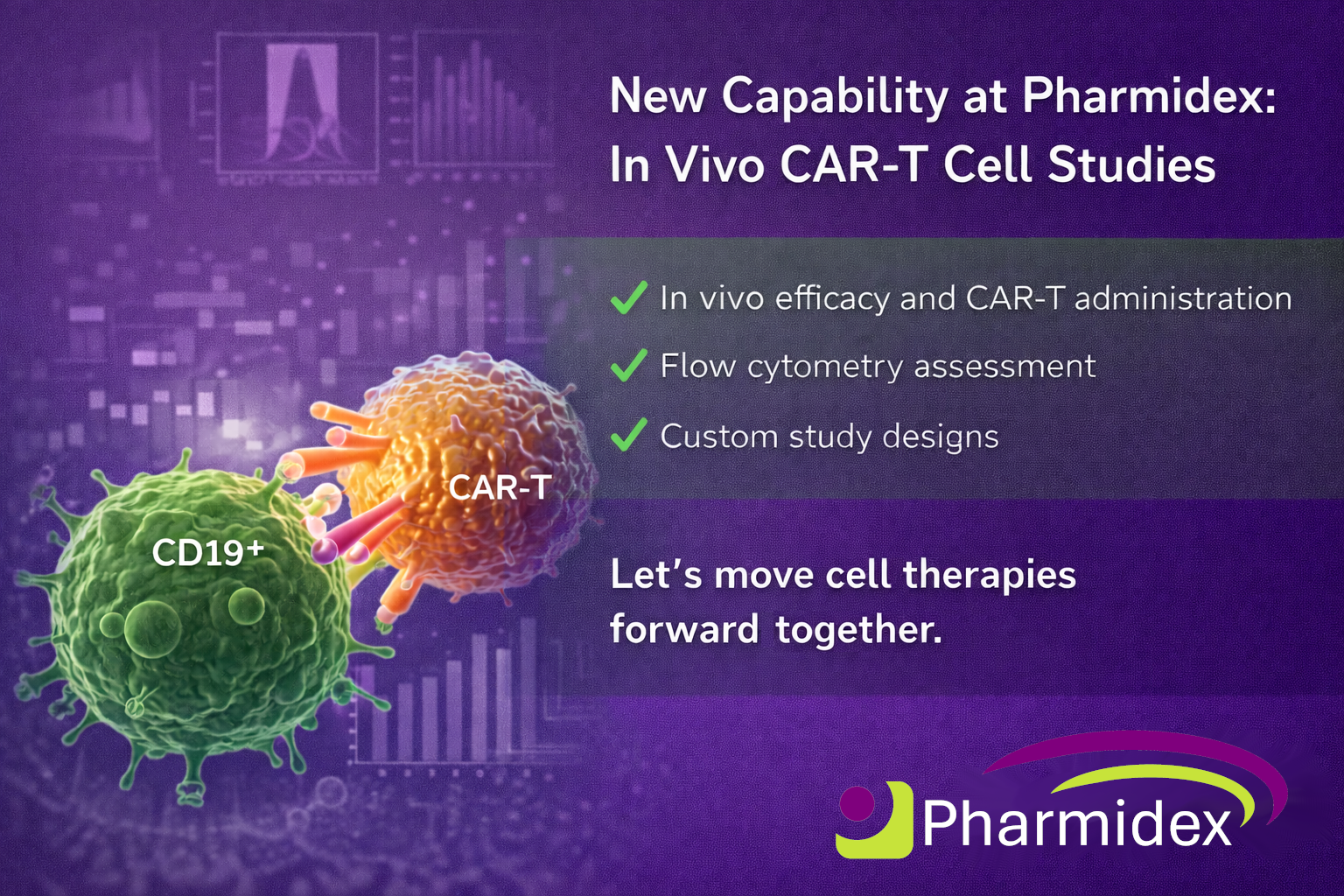
A Consortium led by Intract Pharma has successfully been awarded funding from Innovate UK to develop a scalable manufacturing process for novel oral antibody products using it's proprietary Soteria® technology. Pharmidex are also members of this consortium together with Centre for Process Innovation (CPI) and Quay Pharma.

Intract Pharma Ltd has successfully been awarded funding from Innovate UK, the UK’s innovation agency, to develop a scalable manufacturing process for novel oral antibody products using its proprietary Soteria® technology. The collaborative industrial research project, totalling over £1.4 million, was secured through the Industrial Strategy Challenge Fund for innovative projects in medicines manufacturing. This program will be conducted by a consortium led by Intract, including the Centre for Process Innovation (CPI), Pharmidex and Quay Pharma.
Each member of the consortium brings unique expertise in drug development and manufacture, with an extensive understanding of pharmaceutical formulation. The project seeks to develop a manufacturing process for Intract’s revolutionary Soteria® technology which can be scaled in a commercially viable manner. Soteria® transforms painful and inconvenient injectable therapies into safe, simple oral formulations, and allows targeting and concentration of the antibody at the site of disease in the gut to improve therapeutic efficacy whilst minimising risk of systemic toxicity.
About Pharmidex
Pharmidex provides translational solutions using its world-renowned expertise in CNS/Oncology, drug discovery and ADMET/PK. Founded in the UK in 2002, Pharmidex operates in state-of-the-art facilities in London and Hatfield to provide high quality bespoke experimental data to support drug discovery & development.
About Intract Pharma
Intract Pharma is an oral drug delivery specialist, developing state-of-the-art formulation technologies and unique gastrointestinal models to develop advanced new therapeutics. Since its inception, Intract has pursued innovation in oral drug delivery as a means to improve on current therapies, making them safer and more effective for patients. Soteria® is available for license exclusively from Intract Pharma. For more information visit www.intractpharma.com
About CPI
The Centre for Process Innovation is a UK-based technology innovation centre and part of the High Value Manufacturing Catapult. The company uses applied knowledge in science and engineering combined with state-of-the-art development facilities to enable its clients to develop, prove, prototype and scale up the next generation of products and processes. Its open innovation model enables clients to develop products and prove processes with minimal risk. CPI provides assets and expertise, so customers can demonstrate processes before investing substantial amounts of money in capital equipment and training. New products and processes can be shown to be feasible; on paper, in the lab and in the plant before being manufactured at an industrial scale. By utilising its proven assets and expertise, companies can take their products and processes to market faster. There is no down time in production as all of the process development is completed offsite and CPI’s technology transfer and engineering teams can help companies transfer the product or process into full-scale production at speed.
About Quay Pharma
Quay Pharmaceuticals is experienced in providing a comprehensive out-sourcing service for formulation and analytical development with subsequent clinical trials manufacturing for pharmaceutical and biotechnology companies worldwide. Working as a dedicated, pro-active part of your team we apply strong project management and technical expertise to take products quickly and cost effectively from late stage pre-clinical through to the end of phase II clinical trials. Quay Pharmaceuticals has built a reputation for specialising in the formulation of APIs that exhibit poor solubility and bio-availability and those which require modified or controlled release.
About Innovate UK
Innovate UK drives productivity and economic growth by supporting businesses to develop and realise the potential of new ideas. We connect businesses to the partners, customers and investors that can help them turn ideas into commercially successful products and services and business growth. We fund business and research collaborations to accelerate innovation and drive business investment into R&D. Our support is available to businesses across all economic sectors, value chains and UK regions. Innovate UK is part of UK Research and Innovation. For more information visit www.innovateuk.ukri.org